
FAQs
If you are a current patient, we thank you for entrusting Orthofix with your healing process. We have compiled a list of Frequently Asked Questions (FAQs ) that may be of assistance to you. Please contact us directly by phone or by email.
Next Steps
Doctors may prescribe Bone Growth Therapy, commonly known as bone growth stimulation, for patients who are experiencing bone healing complications or exhibit risk factors that can impede healing. If you are concerned that your fracture or spinal fusion is not healing or if you have reason to believe that you have a risk factor that will make it difficult for you to heal, it is important to speak with your doctor, who can diagnose your condition and prescribe treatment.
Physicians may prescribe a bone growth therapy device when a patient has poor bone healing. Common bone healing risk factors include:
- Smoking/tobacco use.
- Diabetes.
- Obesity.
- Multilevel fusions.
- Medication that depletes bone.
- Spondylolisthesis (Grade 2+).
- Allograft (bone graft) use.
If your doctor prescribes an Orthofix bone growth therapy device, an Orthofix representative will contact you to schedule an appointment – either at the doctor’s office or at your home to explain the benefits of the device, how it works, insurance requirements and to ensure that it is properly fitted.
For a complete guide of what to expect after a device has been prescribed for you, watch our Patient Fitting Video.
Wearing and Operating Your Stimulator
- Yes. TheSpinalStim™, CervicalStim™, and PhysioStim™ devices can be worn over an orthopedic brace or clothing without affecting the PEMF signal to the fracture site or fusion site.
- No. The AccelStim™ transducer requires direct contact with the skin along with coupling gel provided with your device. If you have a cast, please contact Patient Services at 1-800-535-4492 as an accessory may be available.
- SpinalStim™, CervicalStim™, and PhysioStim™ devices
- You won’t feel the PEMF therapy, and the device is lightweight and adjustable for a comfortable fit. It is powered with a rechargeable battery, which allows the unit to be portable. You can sit, stand, sleep, walk, recline, and drive while using your device. With your physician’s approval, you can resume a normal activity level.
- AccelStim ™ devices
- Most patients do not feel anything at the treatment site, while some patients report experiencing a tingling sensation. Please contact Patient Services at 1-800-535-4492 with questions or concerns.
Treatment is based on a daily therapy schedule. Your doctor will prescribe the device for a certain number of hours each day, based on your needs. The Treatment is based on a daily therapy schedule. Your doctor will prescribe the device for a specific treatment time each day, based on your needs. The minimum daily treatment time for:
- The SpinalStim™ device is two hours per day
- The PhysioStim™ device is three hours per day
- The CervicalStim ™ device is four hours per day
- The AccelStim™ device is 20 minutes per day
- Yes. TheSpinalStim™, CervicalStim™, and PhysioStim™ devices treatment sessions can be split up. It is recommended that the treatment sessions be a minimum of 60 minutes. Patients have the flexibility to receive treatment at any time during the day. The device has a built-in 24-hour clock which allows treatment each day between the hours of 12:00 a.m. through 11:59 a.m. (Central Time, unless adjusted for your time zone).
- Yes. TheAccelStim™ device treatment session can be split up into two 10-minute treatment sessions. It is recommended to complete the entire 20-minute session each day.
No. You have the flexibility to receive your treatment at any time during the day. The device has a built-in 24-hour clock which resets daily at 12:00 midnight, Central Time, unless adjusted for your time zone’s instructions.
The healing process itself determines how long you wear a bone growth stimulator, and your physician will closely monitor your progress. Your individual risk factors (such as smoking, multi-level fusion, and graft type) and your compliance in wearing the device will factor into the duration of your treatment. To promote your healing, it is very important that you wear the bone growth therapy device daily as prescribed. Your doctor may require that you bring your unit in on your follow-up visits to check your compliance. Most patients wear the device between three and nine months.
- The SpinalStim™, CervicalStim™, and PhysioStim™ devices use a PEMF technology that produces a waveform that humans can’t sense, so you’ll never feel the treatment. If your device display shows a timer countdown, the unit should be working. If at any time the device stops showing a treatment timer or shows a code starting with the letter E, please contact Patient Services at 1-800-535-4492.
- The AccelStim™ device is working properly when you see the display screen counting down from 20 minutes during each treatment. Your physician will update you on your healing status at follow-up appointments.
One year. If at any time you need a replacement part, please contact Patient Services at 1-800-535-4492.
Orthofix Bone Growth Therapy offers a free recycling program so that patients can properly dispose of their Bone Growth Therapy devices once treatment is completed.
While the battery for Orthofix Bone Growth Therapy devices will last for an average of 10 treatment hours, it is strongly recommended that the device be recharged.
- The SpinalStim™, CervicalStim™, and PhysioStim™ devices will last for an average of 10 treatment hours, it is strongly recommended that the device be charged after completing daily treatment. The device will not deliver treatment while charging.
- The AccelStim™ device can deliver up to 5 treatments when the internal battery is fully charged, it is strongly recommended that the device be charged after completing the daily treatment.
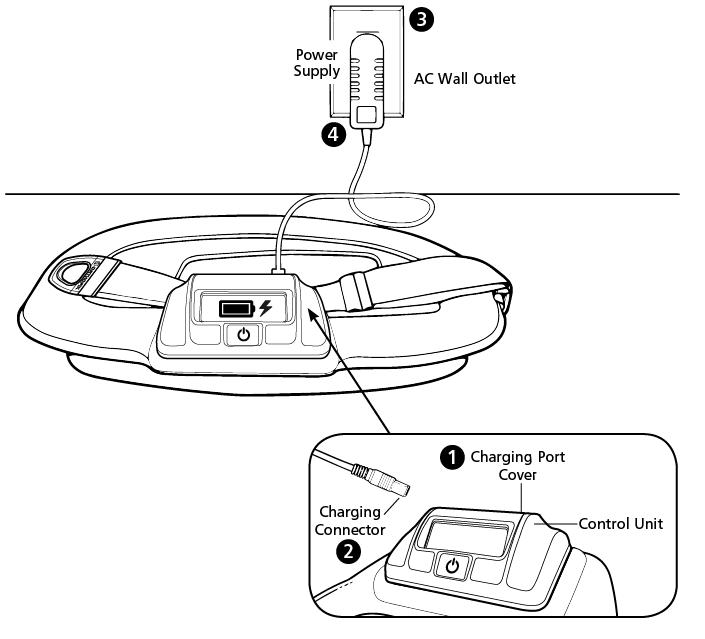
- The SpinalStim™, CervicalStim™ and PhysioStim™ devices can be cleaned by wiping surfaces with a damp, soft cloth (wet with water only). Do not use solvents or expose to excessive moisture.
- Cleaning foam inserts or cushions:
- Hand wash in cold water using a mild laundry detergent (do not dry clean or clean with bleach or other solvents).
- Wring out excess water and pat dry with a clean towel.
- Lay flat until completely dry (do not put in the dryer and do not iron).
- The AccelStim™ device should be used following good hygiene practices and cleaned regularly. Avoid hair, dust, and exposure to direct sunlight. Before cleaning the AccelStim device, make sure that it is switched off and disconnected from the power supply. To avoid potential damage, handle the transducer carefully using the instructions below. and do not drop it. Clean the device thoroughly to help ensure effective treatment.
- Clean the device after each treatment as indicated below:
- Switch off the AccelStim device. Gently wipe with a slightly damp cloth using water or a neutral detergent (such as a household liquid dishwashing detergent).
- Never use any spray products directly on the AccelStim device to avoid the risk of liquid penetration.
- Never pour water or liquids of any type onto the AccelStim device.
- The elastic strap can be washed like ordinary clothing
The unit has expired. Please consult with your physician.
When traveling by air, it is recommended to pack stimulators with checked luggage. If taken onboard the airplane, it should be turned off when passing through security screening equipment, as the device could be damaged. The instruction manual should be taken with you to quickly and easily identify the device for security personnel.
Using your STIM onTrack mobile app
It is a U.S. Food and Drug Administration (FDA) approved software application for mobile devices that works with Orthofix CervicalStim™, SpinalStim™, and PhysioStim™ bone growth therapy devices. It provides tools to help patients adhere to their bone growth therapy device prescriptions and achieve the desired clinical outcome.
Your bone growth therapy device records the duration and frequency the device is used. The device does not collect or record patient identifiable information.
STIM onTrack collects the duration and frequency the bone growth therapy is used to ensure compliant treatment with what the doctor has prescribed. The mobile app does not collect or record patient identifiable information.
Collecting the duration and frequency the device is used, can help ensure compliant treatment with what the doctor has prescribed.
The data recorded by your mobile application may be reviewed by Orthofix and your health care provider. The data collected is not shared with third parties.
- Orthofix Privacy and HIPAA Notice of Privacy Practices can be accessed here: www.bonegrowththerapy.com/privacy-policy/
- For additional questions, please contact the Privacy Office at privacy@orthofix.com or call 800-535-4492.
- In order for the STIM onTrack mobile app to connect to the bone growth therapy device, the device must be turned on and providing treatment.
- Be sure to check that the Bluetooth function on your Apple or Android device is turned on.
- If you have an Android device, the location must be turned on.
The STIM onTrack mobile app is an optional accessory of your bone growth therapy device, and while you are not required to download the app, it is strongly recommended you use it to track your treatment progress and have access to educational resources.
- It roughly takes up about as much as one song.
- WIFI availability allows the app to upload data when connected.
- Treatment Time remaining to complete a treatment session for the day.
- A calendar displays of your treatment and usage data.
- Total Days of Treatment.
- Days since Start of Treatment.
- Compliance Percentage.
- Prescribed Treatment Time.
- Visit: www.bonegrowththerapy.com/therapies/stim-ontrack-app/ to see more.
Physicians can view adherence to your prescribed treatment.
Billing and Insurance Coverage
After your physician determines that you would benefit from a bone growth therapy device, he or she provides Orthofix with a written prescription and other information required by your insurance provider to determine whether the device is covered under your plan. Orthofix then works with your insurer to determine coverage before you receive the device. This process can take a few days or even several weeks.
Insurance policies are different depending on the plan you have chosen. If coverage guidelines are met, the bone growth therapy device is accepted and approved by the majority of private and public health plans, including Medicare, Medicaid and worker’s compensation plans. Some plans include a deductible, co-payment, or other co-insurance amount. Please see “Is there financial responsibility for patients?” below.
Health insurers, including Medicare, typically cover only those items and services which are determined by their policy to be “reasonable and necessary” for treating specific medical conditions. To determine medical necessity, health insurers require providers such as Orthofix to provide information about your diagnosis to determine whether the device is covered by your insurance plan.
Even if an item is considered medically necessary and, therefore, covered by insurance, some health insurers require you to pay a portion of the cost. These costs could include a deductible, co-payment or other co-insurance amount. For Medicare patients, the co-insurance amount for a bone growth stimulator is generally 20% of the Medicare allowable amount. For patients with other health insurance, the co-insurance amount varies by insurer.
Yes. If your insurance has determined that you have a coinsurance/deductible, you will receive a bill with instructions for payment. Please visit our contact page for details.
If this happens, the claim will be pursued by our appeals processing department on your behalf. If all appeals are exhausted and your contracted provider has denied medical necessity, you may receive a claim. If you have not already made prior payment arrangements and you receive a claim, please contact Patient Services at 1-800-535-4492 to discuss your payment options and/or arrangements.
An ABN is an Advance Beneficiary Notice of Noncoverage for Medicare patients. This document gives patients advance notice that Medicare may not pay for the item prescribed by the physician for their condition. The ABN informs you of your financial responsibility if you choose to receive the device. If an ABN is required for your specific situation, you will be asked to sign it before you receive the bone growth therapy device.
Like the ABN, the Advance Notice of Noncoverage (ANN) gives non-Medicare patients advance notice that their insurer may not pay for the item prescribed by the physician for their condition. The ANN informs you of your financial responsibility if you choose to receive the device. If an ANN is required for your specific situation, you will be asked to sign it before you receive the bone growth therapy device.
Please contact our Patient Care Billing Specialists at 1-866-543-9340 to discuss payment options.
Orthofix is required by law to collect a patient’s co-insurance or other amount owed for the bone growth therapy device. Please contact Orthofix with your billing questions (800-535-4492).
Please contact our Patient Care Billing Specialists at 1-866-543-9340 to discuss payment options and questions about financial assistance.
Do not be alarmed if you receive a denial from your insurance company. You have the right to appeal!
- Coverage denials are frequently reversed in the patient’s favor when appealed.
- While many disputes are resolved after the first appeal, others require escalation to second level or through an external review process.
- The Affordable Care Act (ACA) ensures the patient’s right to appeal an adverse determination to their health plan and, if necessary, to a third-party reviewer.
- The health plan cannot raise premiums or drop coverage because of an appeal.
Your health plan has an internal appeals process that includes up to two separate levels, and a third external review option. It is important to note that each review level requires additional information be submitted. This additional information may include lab reports, office notes or scientific literature not previously reviewed.
- First Level – This level is usually reviewed by a Medical Director to determine whether the claim actually does meet insurance guidelines and was denied in error.
- Second Level – A second Medical Director, not involved in the first level of review, determines whether the claim meets guidelines.
- Independent/External Review – A physician, who is board certified in the same specialty as the prescribing physician, and not affiliated with or influenced by your insurance plan, reviews the information and determines whether the claim meets guidelines.
Under the Affordable Care Act, patients have the right to appeal a health insurance company’s decision to deny payment for a claim, including the denial of a claim for an Orthofix bone growth therapy device. However, it is imperative that patients check their own health plans to follow the policies and procedures applicable to appeals. Your state may have Consumer Assistance Programs that can help you file an appeal or request a review of your health insurance company’s decision if you are not sure what steps to take. Your insurance company should have provided you with information about how to file an appeal and the appeals process when you were enrolled in coverage, and there may be information about the process on the plan’s website. Visit LocalHelp.HealthCare.gov to find help in your area. For additional information, you may also call your health plan or state insurance regulator.
Appeals are most likely to go in your favor when the information is concise, complete, and details are based on fact rather than emotion. Be sure to retain original records for your files while submitting copies to the health plan for the appeal. Orthofix may have many of these documents and can assist you in compiling your Appeal packet upon request.
Getting started:
- Identify the reason for your denial, if any forms are required for the Appeal, the address to file your appeal and any timelines for filing. This will be included in the denial notice issued by your health plan.
- Prepare your Appeal Letter.
Important information to also enclose with your Appeal:
- Physician’s Letter Of Medical Necessity.
- Office notes on your condition or the procedure performed (operative note).
- Supporting records of associated risks to bone healing. Risks to fusion success could include smoking, diabetes, osteoporosis, bone depleting medications, etc.
- Peer-reviewed or medical literature from professional journals or magazines regarding the stimulator prescribed.
- Submit your completed packet via certified mail with a request for a return receipt.
How do I follow up on my appeal?
- If you have not yet received your bone growth therapy device, the health plan has 30 days to respond to your appeal.
- If you have already received your bone growth therapy device, the health plan has 60 days to respond to your appeal.
- Remember, you can escalate your appeal if the denial is upheld on the first appeal.
Safety
Yes. Orthofix Bone Growth Therapy devices were specially designed with your safety in mind. Over 1,000,000 Orthofix patients have worn our devices to increase the probability of fusion success or to heal a non-union fracture.
Yes. SpinalStim™, CervicalStim™, PhysioStim™, and AccelStim™ devices are FDA approved as a Class III medical devices. The PhysioStim device was approved by the FDA in 1986, the SpinalStim device was approved by the FDA in 1990, the CervicalStim device was approved by the FDA in 2004, and the AccelStim device was approved by the FDA in 2022.
Indication
The PhysioStim™ device is indicated for the treatment of an established nonunion acquired secondary to trauma, excluding vertebrae and all flat bones, where the width of the nonunion defect is less than one-half the width of the bone to be treated. A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing.
Contraindication
Use of this device is contraindicated where the individual has synovial pseudarthrosis.
Warnings
- The safety and effectiveness of the use of this device on individuals lacking skeletal maturity has not been established.
- In the presence of a malaligned nonunion, careful consideration of the use of this device must be undertaken on an individual basis, as treatment with this device is not intended to alter or affect the degree of malalignment.
- Demand type pacemaker operation may be adversely affected by exposure to pulsed electromagnetic fields. Physicians should not prescribe a PhysioStim for application which may place the treatment transducer in close proximity to the pacemaker. Further screening by the attending cardiologist is recommended (such as with an electrocardiogram).
- Animal studies conducted to date do not suggest any long-term adverse effects from the use of this device. However, long-term effects in humans are unknown.
- The safety and effectiveness of this device on individuals with a nonunion secondary to, or in connection with, a pathological condition has not been established.
Precautions
- Nonunion fractures with gaps in excess of 1 centimeter (cm) have not been evaluated.
- Although animal reproductive studies performed with this device demonstrated no adverse findings, the safety of use of this device during pregnancy and nursing in humans has not been established.
- This device should not be used if there are mental or physical conditions which preclude patient compliance with physician and device instructions.
Adverse Effects
Rare instances of reversible minor discomfort have been reported. They were: cumbersome or uncomfortable, tingling or pain and minor skin rash.
Indication
The SpinalStim™ device is a noninvasive electromagnetic bone growth stimulator indicated as a spinal fusion adjunct to increase the probability of fusion success AND as a nonoperative treatment for salvage of failed spinal fusion, minimum nine months postoperative.
Contraindication
Cardiac pacemakers may be adversely affected by exposure to PEMF. Use of this device is contraindicated where the individual has an implanted cardiac pacemaker.
Warnings
- Although animal teratological studies performed with the device demonstrated no adverse findings, the safety of use of this device during pregnancy and nursing in humans has not been established.
- The safety and effectiveness of the use of this device on individuals lacking skeletal maturity have not been established.
- Animal studies conducted to date do not suggest any long-term adverse effects from the use of a similar device. However, long-term effects in humans are unknown.
Precautions
- This device should not be used if there are mental or physical conditions which preclude compliance with the physician and device instructions.
- This device has not been evaluated in treating patients with the following conditions: osseous or ligamentous spinal trauma, spondylitis, Paget’s disease, moderate to severe osteoporosis, metastatic cancer, renal disease, and uncontrolled diabetes mellitus.
- The results of premarketing data from the randomized double-masked cohort indicate that inconsistent users (defined as those patients that used the device for less than an average of two hours per day) had success rates similar to those in the placebo group. Therefore, the use of the device for less than the minimum recommended usage may result in lower success rates.
Adverse Effects
Rare instances of reversible minor discomfort have been reported. They were: cumbersome or uncomfortable, minor tingling or pain, minor skin rash, insomnia, fainting, nausea/diarrhea, and polymenorrhea.
Indication
The CervicalStim™ device is a noninvasive, pulsed electromagnetic bone growth stimulator indicated as an adjunct to cervical fusion surgery in patients at high risk for non-fusion.
Contraindication
There are no known contraindications for the CervicalStim device as an adjunct to cervical spine fusion surgery.
Warnings
- Do not use CervicalStim device if you have a cardiac pacemaker or defibrillator because it may interfere with the operation of your pacemaker or defibrillator. If you use the CervicalStim device and it affects your pacemaker or defibrillator, it may injure your heart. Consult your cardiologist.
- Remove the CervicalStim device prior to any imaging procedures (e.g., CT scan, MRI, etc.). If you wear the CervicalStim device during these procedures, you could be injured, the imaging being produced may be ruined, and/or the CervicalStim device could be damaged.
Precautions
- Avoid using the CervicalStim device if you do not understand the instructions your doctor has given you. If you use the CervicalStim device incorrectly, it may harm you or may not help your healing process.
- The CervicalStim device has not been evaluated in treating patients with the following conditions: osseous or ligamentous spinal trauma, spondylitis, Paget’s disease, moderate to severe osteoporosis, metastatic cancer, renal disease, rheumatoid arthritis, uncontrolled diabetes mellitus, patients prone to vascular migraine headache, seizure, epilepsy, thyroid conditions or neurological diseases.
- Animal reproductive studies performed with this device did not show any harmful effects in animals. However, the safety of this device for use on patients who are pregnant or nursing has not been established.
Adverse Effects
Adverse effects may be experienced when using the CervicalStim device. These adverse effects may include: increased pain, numbness and tingling, headache, migraines and nausea. These effects may or may not be directly related to the use of the CervicalStim device. Any adverse effects that are related to the CervicalStim device should stop when you discontinue use.
- Indication
- The AccelStim™ device is indicated for the non-invasive treatment of established nonunions excluding skull and vertebra, and for accelerating the time to a healed fracture for fresh, closed, posteriorly displaced distal radius fractures and fresh, closed or Grade I open tibial diaphysis fractures in skeletally mature adult individuals when these fractures are orthopedically managed by closed reduction and cast immobilization. A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing.
- Contraindication – There are no known contraindications for the AccelStim device.
- Warnings
- Fractures with post-reduction displacement of more than 50% (i.e., fractures in which the opposing broken bone ends are out of alignment by more than one half of the width of the bone).
- Pathological fractures due to bone pathology or malignancy (fractures due to disease).
- Pregnant or nursing women.
- Individuals with thrombophlebitis (blood clot in a vein), vascular insufficiency (poor blood supply), abnormal skin sensitivity (very sensitive skin), sensory paralysis (lack of sensation), alcoholism and/or nutritional deficiency.
- Individuals receiving steroid, anticoagulant, and prescription nonsteroidal anti-inflammatory medications.
- Calcium channel blocker and/or diphosphonate therapy. Individuals using these
- therapies were excluded from the studies because of the possible effects of these therapies on bone metabolism.
- Nonunions of the vertebra and the skull.
- Individuals lacking skeletal maturity.
- Fresh fracture locations other than the distal radius (end of the large bone in the forearm) or tibial diaphysis (middle 80% of the large bone in lower leg).
- Fresh fractures that are open Grade II or III (fractures with large wounds), or that require surgical intervention with internal or external fixation (screws and/or plates used to hold your broken bones in place), or that are not sufficiently stable for closed reduction and cast immobilization (manipulation of the fracture without surgery).
- The AccelStim device is MR Unsafe. The device presents a projectile hazard in this environment.
- The device should not be used over skin that is infected or is not intact, if scarring or blood is evident at the application point, or in the presence of other local substances or abnormal tissues that may affect the acoustic signal such as inflammation (rash) hematoma, or abscess. The impact of such soft tissue abnormalities within the effective radiating area of the transducer has not been studied by any manufacturer.
- Precautions
- The AccelStim device will not correct or alter post-reduction (when your fracture is initially set and placed in a cast) aspects of a fracture such as displacement, angulation or malalignment.
- The transducer, strap and gel are not sterile and placement on an open wound is not advised.
- he operation of active, implantable devices, such as cardiac pacemakers, may be adversely affected by close exposure to the AccelStim device. The physician should advise the patient, or other person in close proximity during treatment, to be evaluated by their attending cardiologist or implant physician before starting treatment with the AccelStim device.
- The cords pose a risk for strangulation. Keep out of reach of children.
- Cell phones, televisions, and other devices using radio frequency identification (RFID) readers, electronic security systems (e.g., metal detectors, electronic article surveillance), near-field communications (NFC) systems, wireless power transfer and unique medical emitters such as electrocautery, electrosurgical units, and diathermy equipment may cause interference. Don’t use the AccelStim device closer than 15 cm (6 inches) from these electromagnetic (EM) emitters.
- The safety and effectiveness of the AccelStim device for use of more than one daily 20-minute treatment period has not been studied.
- When choosing a treatment site, ensure that the site selected allows for full contact of the transducer face with the skin. Failure to do so may result in the transducer being only partially coupled to the skin. This may reduce the effectiveness of the AccelStim device in treating the fracture.
- Only the region of the fracture within the effective radiating area (3.5 cm2) of the transducer is likely to benefit from the AccelStim device’s treatment. Therefore, the physician should take care in appropriately placing of the device over the fracture site.
- Placement of the transducer directly over internal fixation may result in the treatment signal being partially or fully blocked and may reduce the effectiveness of the AccelStim device in treating the fracture.
- When choosing a treatment site, the transducer shall be positioned such that the ultrasound beam is not impeded by any internal fixation which is directly in line with the fracture site (i.e., not directly over metal plating). This may require placement of the transducer on the opposite side of the limb or perpendicular to the fracture line. Correct placement should be confirmed using radiographic and/or anatomical markers by a health care provider during the fitting of the device. The AccelStim device’s site of application should be marked onto the patient’s skin with an indelible marker to guide future transducer placements.
- Adverse Effects
- Unlike conventional (physical therapy) ultrasound devices, the AccelStim device is incapable of producing harmful temperature increases in body tissue. 42 The output intensity of the device your patient receives is 30mW/cm 43 and is typically only 1% to 5% of the output intensity of conventional therapeutic ultrasound devices. The ultrasound intensity is comparable to diagnostic ultrasound (1 to 50 mW/cm 2), such as the intensities used in obstetrical sonogram procedures (fetal monitoring). In addition, there is no evidence of nonthermal adverse effects (cavitation). While no device related adverse reactions or medical complications were reported in the referenced clinical studies (see “Clinical Studies” section in this manual), there are several potential adverse events associated with the use of this device. In case you experience any pain, discomfort or other unwanted effects related to the use of the device, stop using the device and contact Patient Services and/or your physician.
The safety of use of this device during pregnancy and nursing in humans has not been established.
- Using a SpinalStim™ device with an implanted cardiac pacemaker is contraindicated, while it’s a warning with the CervicalStim™ and PhysioStim™ devices. Demand type pacemaker operation may be adversely affected by exposure to pulsed electromagnetic fields. Physicians should not prescribe a SpinalStim, CervicalStim, or PhysioStim device for application which may place the treatment transducer in close proximity to the pacemaker. Further screening by the attending cardiologist is recommended (such as with an electrocardiogram). It’s important to consult your cardiologist, who can run tests to determine whether the device will affect your specific pacemaker model.
- The AccelStim™ device has no known contraindications, however, the operation of active, implantable devices such as a cardiac pacemaker may be adversely affected by close exposure to the device. If you have a pacemaker, talk with your doctor or cardiologist to discuss if the AccelStim device is right for you.
- The SpinalStim™, CervicalStim ™, and PhysioStim™ device’s effect of PEMF treatment was studied in patients with skeletal maturity and has not been studied in patients lacking skeletal maturity. Your doctor will determine when use is medically appropriate.
- The AccelStim™ device effects of LIPUS treatment were studied in patients that are skeletally mature adult individuals only. Your doctor will determine when use is medically appropriate.
Orthofix Patient Services
(800) 535-4492 toll free
(7:00 a.m. – 7:00 p.m. CST)
(800) 445-1923 fax
PatientServices@Orthofix.com